Parenteral Solution Manufacturing Facility
Introduction
We specialize in the development and production of parenteral solutions, operating under the highest standards of sterility and quality control. Parenteral solutions are sterile, pyrogen-free preparations designed for administration via through the skin, internal or other boundary tissues. This dosage form enables immediate systemic availability by bypassing the gastrointestinal tract, ensuring precise and controlled delivery.

Send Enquiry
Types of Parenteral Solutions
Vials
Manufactured in glass or plastic containers, sealed with a rubber stopper and aluminium cap, these vials are designed for multiple dosing applications while maintaining sterility and integrity.
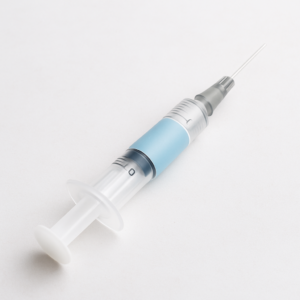
Pre-filled Syringes
These are sterile syringes pre-loaded with a fixed dose of medication, designed to ensure precision, safety, and convenience in parenteral administration.
Small-V Parenterals
Sterile parenteral solutions packaged in containers of 100ml or less, designed for precise dosing and diverse precision applications.
Lyophilized Products
These are freeze-dried powders packaged under sterile conditions, requiring the addition of a diluent before injection. This format helps maintain stability and shelf life of sensitive formulations.



Packaging, Labelling & Special Content
Primary Packing
Glass Containers– Type I borosilicate glass ampoules and vials are widely used for their chemical stability, durability, and inertness, ensuring product integrity.
Plastic Containers– For large-volume parenterals (LVPs), IV bags are produced using polypropylene (PP), polyethylene (PE), or polyvinyl chloride (PVC), offering flexibility and sterility.
Rubber Stoppers– Vials are sealed with butyl or halo-butyl rubber stoppers, engineered to maintain tight closure and withstand processes like lyophilization.
Prefilled Syringes– Complex assemblies featuring glass barrels, rubber plungers, and protective needle shields, designed for convenience, sterility, and precision dosing.
Box Packaging
Cardboard Trays & Boxes- Designed to securely hold vials and ampoules, these packs provide essential physical protection during handling and distribution.
Package Inserts- Every pack includes mandatory informational leaflets for healthcare professionals, ensuring safe and compliant use of the product.
Mandatory Content- Labels and inserts clearly state: Product Name, Strength per volume, Batch Number, Manufacturing & Expiry Date, Route of Administration, Fill Volume, Manufacturer details, Warning Statements, and Storage Instructions.
Technical Details- Additional data such as osmolarity, pH values, and complete solution composition are often included to meet regulatory requirements.
Special Content- May highlight key notes such as “Single-use only,” “Lyophilized Powder – See Reconstitution Instructions,” “Preservative-Free,” or QR Codes for digital traceability.
Manufacturing & In-Process Control
Primary Packing
Dispensing
All ingredients are precisely weighed in controlled environments and logged in the Dispensing Record for traceability.
Solution Preparation
Formulation is compounded in stainless steel vessels using WFI under strict quality standards.
Filtration
The solution undergoes sterile filtration through a 0.22 micron filter into a holding vessel—an essential step to ensure sterility.
Aseptic Filling & Sealing
Filling is performed in an ISO 5 cleanroom using isolators or RABS, with operators in full sterile gowning.
Ampoules: Sealed by fusion.
Vials/Bags: Stoppered and secured with an aluminium crimp seal.












