Quality Compliance : Precision, Safety & Global Standards
Quality Compliance & Regulatory Excellence
We place quality and compliance at the core of our operations, ensuring every product meets stringent global safety, efficacy, and documentation standards. Our facilities integrate certified third-party testing, robust quality management systems, and experienced regulatory teams to maintain audit-ready operations at all times. With compliance frameworks tailored for 30+ international markets, we deliver export-ready bioactive naturals, plant-based functional formula, superfood complex, specialty formulation, signature care line, radiance & glow essentials. By combining technical precision with regulatory excellence, we empower brands to achieve market confidence and long-term success worldwide.

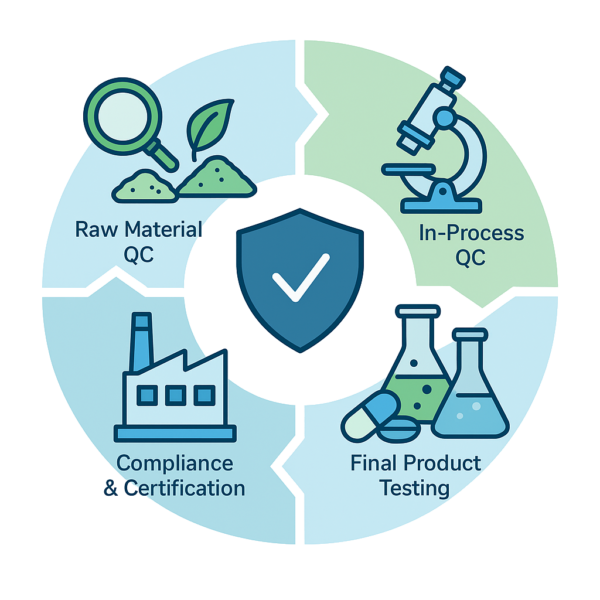
Stepwise Quality Control Stages
Raw Material Quality Assurance
- Authentication & Purity Testing – Using HPLC, GC-MS, and FTIR for botanical and chemical profiling.
- GACP-Certified Sourcing – Partnering with farms and suppliers following Good Agricultural & Collection Practices.
- Contaminant Screening – Heavy metals, pesticides, mycotoxins, and microbiological testing.
In-Process Quality Checks
- Continuous monitoring of critical control points (CCPs) during manufacturing.
- Sampling and analysis at blending, filling, and packaging stages.
- Real-time corrective actions to prevent deviations.
Finished Product Verification
- Potency & Stability Testing – Ensuring label claim accuracy and validated shelf life (up to 5 years).
- Safety Evaluation – Allergen control, microbial limits, and preservative efficacy.
- Packaging Integrity – Leak testing, seal validation, and barrier performance checks.

Certifications & Regulatory Compliance
Global Certifications
- WHO-GMP – World Health Organization Good Manufacturing Practice.
- ISO 22000 – Food Safety Management.
- ISO 22716 – Cosmetic Good Manufacturing Practice.
- HACCP – Hazard Analysis & Critical Control Points.
- Kosher Certification.
- Organic (USDA/EU/India) Certification.
International Regulatory Alignment
- US FDA 21 CFR 111/210 for dietary supplements and cosmetics.
- EU Regulations (EC 1223/2009) for cosmetic products.
- TGA (Australia), GCC, ASEAN compliance for export markets.
Advanced Quality Compliance Infrastructure
Laboratory Capabilities
Analytical Chemistry– Our analytical chemistry laboratories are equipped to deliver precise active ingredient quantification. Every test is conducted with validated methods to ensure accuracy, consistency, and compliance with international regulatory standards.
Microbiology– Our microbiology labs specialize in the detection and identification of pathogens. With advanced testing protocols, we help safeguard product safety and ensure alignment with stringent quality regulations.
Stability Studies– Our stability chambers support both accelerated and real-time studies. These facilities provide reliable data for shelf-life determination, product performance validation, and global compliance readiness.
Digital Compliance Systems












